Abstract
Background:
Positron emission tomography-computed tomography (PET-CT) is used for staging and response assessment in classical Hodgkin lymphoma (cHL) and for evaluation and management of refractory/relapsed Hodgkin lymphoma (RRHL). According to the World Health Organization's Global Atlas of Medical Devices 2017 report, 92-95% of lower-middle and low-income countries have no PET/CT unit, and only 3% of upper-middle income countries have 1 PET scanner/million people, versus 29% of high-income countries. Real-world data on PET scan use in cHL and RRHL outside Europe and North America are limited. The B-CD30+ HOdgkin Lymphoma International Multi-center Retrospective Study of Treatment Pract Ices and Out Comes (B-HOLISTIC) study assessed real-world treatment practices and clinical outcomes in patients with stage IIB-IV cHL and RRHL in countries outside Europe and North America and imaging results are presented here.
Methods:
The B-HOLISTIC study retrospectively reviewed patients (≥18 years) with stage IIB-IV cHL or RRHL between 2010 and 2013. Patients initially diagnosed with cHL who progressed to RRHL during the study were included in both groups. Details of PET and CT scans at baseline and during/end of frontline/salvage treatment, were reported in both groups.
Results:
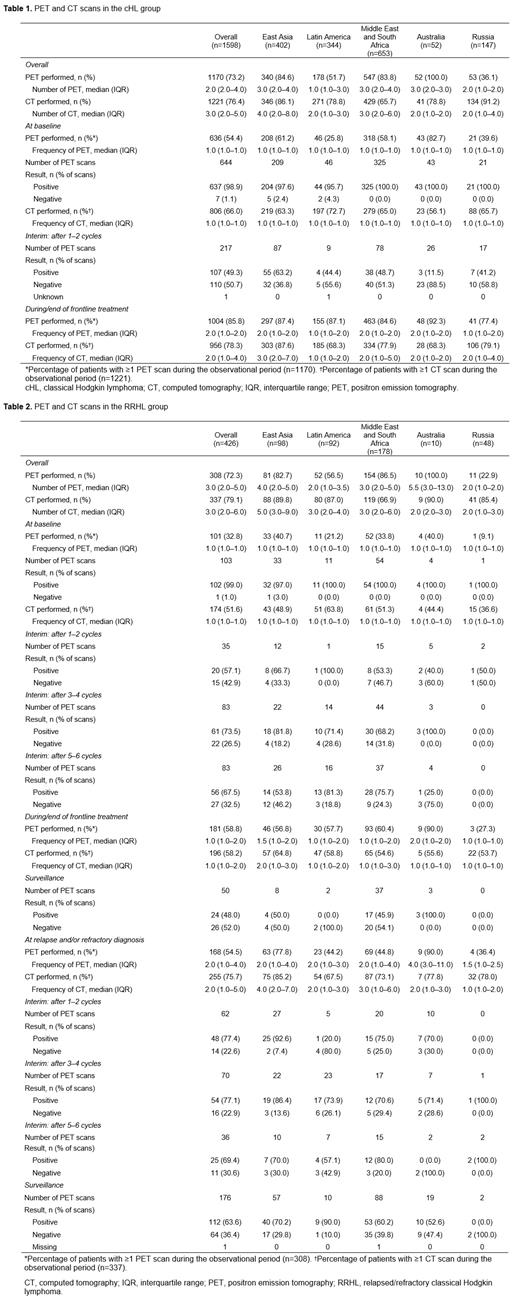
Overall, 1703 patients (cHL: 1598, RRHL: 426, both: 321) were enrolled (East Asia: 426, Latin America: 366, Middle East and South Africa: 694, Australia: 56, Russia: 161). Median follow-up was 5.4 and 4.4 years in the cHL and RRHL groups, respectively. PET and CT results for cHL and RRHL groups are shown in Tables 1 and 2, respectively.
The proportion of patients with PET scan was 73.2% in the cHL group with a median (interquartile range [IQR]) frequency of 2.0 (2.0-4.0) and 72.3% in the RRHL group with a median (IQR) frequency of 3.0 (2.0-5.0). In both groups, the proportion of PET scans at baseline was lower (cHL: 54.4%; RRHL: 32.8%) than during/ end of frontline treatment (cHL: 85.8%; RRHL: 58.8%) and at relapse/refractory diagnosis (54.5%). In contrast, the proportion of CT scans was higher (cHL: 76.4%; RRHL: 79.1%), particularly at baseline (cHL: 66%; RRHL: 51.6%). The highest proportion of PET scans was reported in Australia and lowest in Russia. The highest proportion of CT scans was in Russia in the cHL group and in Australia and East Asia in the RRHL group, while the lowest was in Middle East and South Africa in both groups. The frequency of interim PET scans was low in both cHL and RRHL groups, and were rarely used in cHL surveillance. In the RRHL group, interim PET scans during the frontline therapy were higher after cycles 3-4 and 5-6 than after cycles 1-2. Deauville 5-point scale (5-PS) was used for PET assessment at interim treatment cycles, end of frontline/salvage treatment, relapse, and surveillance in both groups with increased scans reporting a 5-PS rating towards the end of treatment and surveillance. However, its overall use was suboptimal with a minority (<50%) reporting a specific Deauville score.
Discussion and Conclusion:
This study provides real-world evidence on PET use in cHL and RRHL outside Europe and North America, which is suboptimal. Although PET is part of standard care for cHL now, during 2010-2013 it was more commonly used only in RRHL, as reflected in the higher PET use in RRHL than cHL in this study. Lower overall PET use than CT and the regional differences may reflect the comparatively limited access and availability of PET, especially in low-income countries. Lower PET use at baseline may have been due to low accessibility of PET at the beginning of the study, which improved over time. Higher PET use at the end of frontline treatment in cHL is in line with the literature, and suggestive of its recognized benefits in guiding further treatment if used early in treatment. Lack of data on Deauville ratings could be because its use was uncommon during the time of the study. However, the increased Deauville score reporting towards the end of the study suggests a trend for its use. Overall, the low interim PET use, regional differences, and lack of data on use of Deauville rating in this study, suggest an existing gap in real-world practice and highlight the global inequities in access to PET. These findings suggest the need for upscaling numbers and access to PET scanners outside Europe and North America through careful planning and in-depth assessment of socioeconomic, demographic, and epidemiological circumstances of each country.
Ferhanoglu: Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Zerga: Takeda: Honoraria; Sandoz: Honoraria; Bristol Myers Squib: Honoraria; Jansen: Honoraria; Roche: Honoraria. Kim: Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeyondBIO: Consultancy, Membership on an entity's Board of Directors or advisory committees; Boryung: Consultancy, Membership on an entity's Board of Directors or advisory committees; Hanmi: Consultancy, Membership on an entity's Board of Directors or advisory committees; GI CELL: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca-KHIDI: Research Funding; AstraZeneca/MedImmune: Consultancy, Membership on an entity's Board of Directors or advisory committees. Karduss: Takeda: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Novartis: Membership on an entity's Board of Directors or advisory committees. Kwong: Amgen: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding. Wu: Takeda: Current Employment, Current equity holder in publicly-traded company. Huang: Takeda: Current Employment, Current equity holder in publicly-traded company. Hertzberg: Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees.